Electroosmotic flow (EOF) is an important transport phenomenon in all electrophoretic separation processes that occurs whenever a voltage is applied to a liquid system in contact with a charged solid surface.
In the CZE, the reason for the occurance of EOF is the use of the “fused-silica” capillaries: The silanol groups SiOH of the quartz glass can, depending on the pH of the adjacent solution, be in the form of SiOH 2+ (pH <2.5), neutral SiOH or SiO2 groups (pH> 2.5) are present at the surface [CRC Handbook of Chemistry and Physics, CRC Press, Boca Raton, 67th ed (1986)]. Most often the electrolyte solutions that are used have a pH in the range of 4-10, which means that the inner capillary surface is negatively charged. As a result, it is preferable to attach cations from the aqueous solution to the capillary wall to balance the surface charge. This monomolecular layer (Helmholtz layer) is held by electrostatic forces on the capillary wall. The rigid layer is followed by a diffuse double layer, also referred to as the Gouy-Chapman or Debye-Hückel layer. The potential difference (zeta potential) present within this diffuse double layer is the actual reason for the formation of an EOF, because the positive charge of the “liquid column” in the capillary causes the entire solution to be “pumped” towards the cathode. This results in a uniform flow over the entire length of the capillary with a piston-shaped radial flow profile which, in contrast to a hydrodynamic flow with a parabolic flow profile, exerts no influence on the zone width and therefore does not lead to a deterioration of the separation efficiency.
By overlaying one’s own migration with the EOF, the analyte ions have an effective migration rate that is composed of the velocity vectors of the individual ions and the EOF (see Fig.).
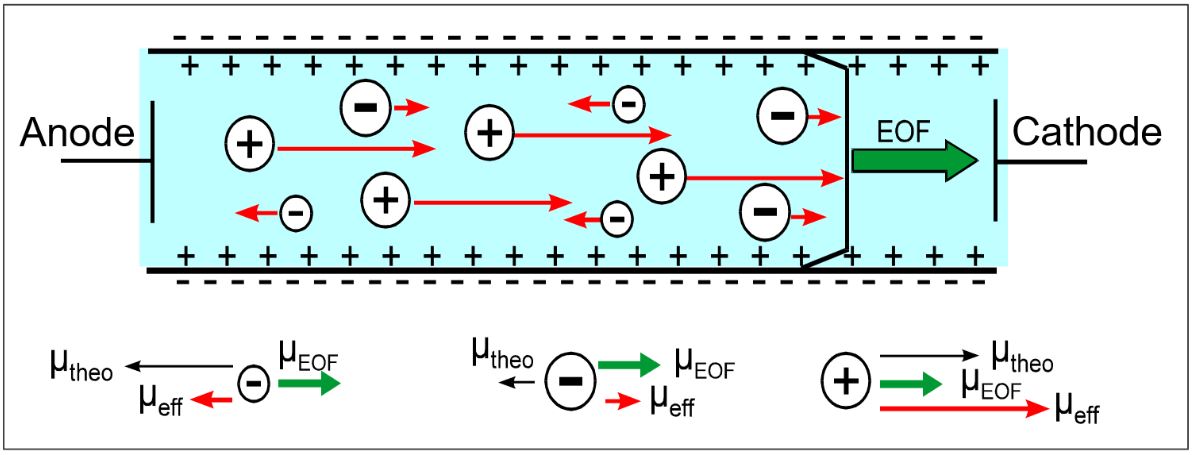
The influence of the EOF on the velocity of the ions
As a result, the analysis times for cations are shortened, while anions can be drastically slowed down or even reversed in their direction of motion due to the opposing EOF. The effective mobility of the individual components can be determined by supplementing an EOF term from an electropherogram:
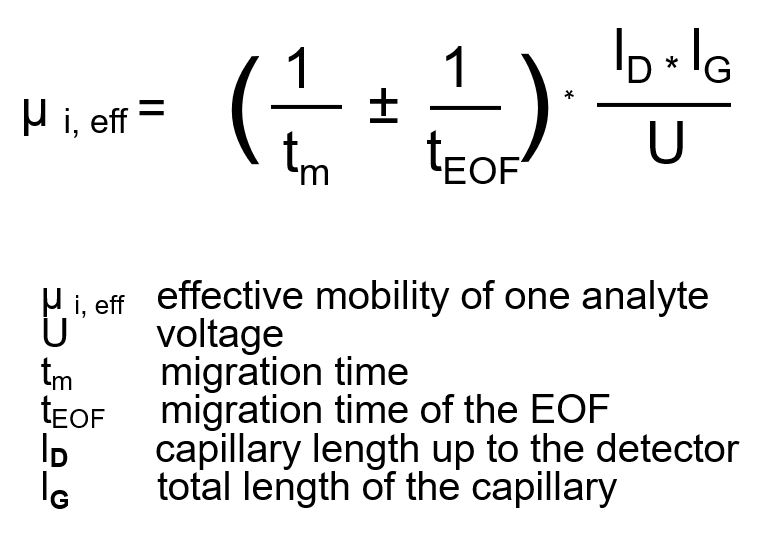
Source: Dissertation, Jana Boden, TU Darmstadt 1996, page 8.